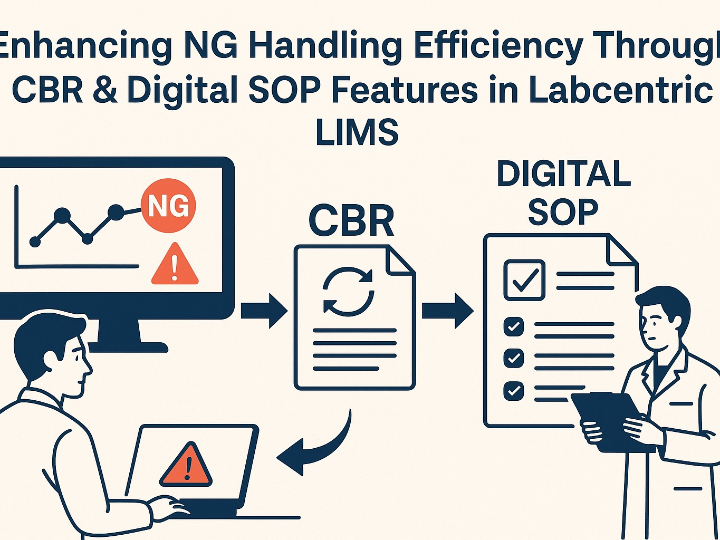
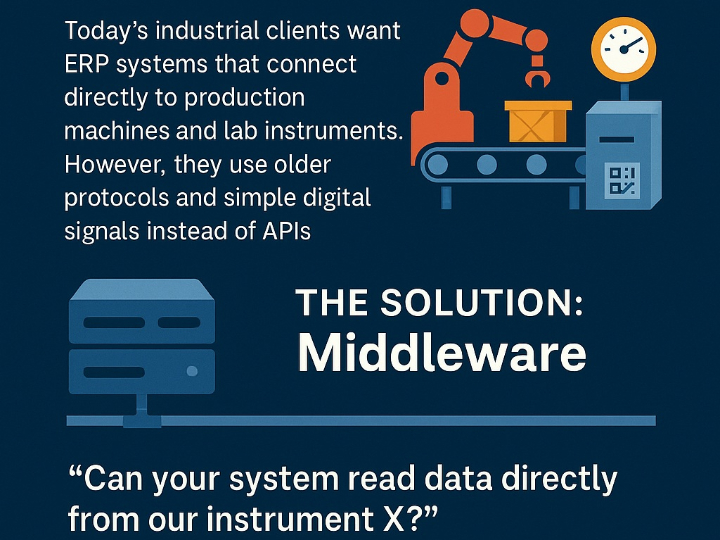
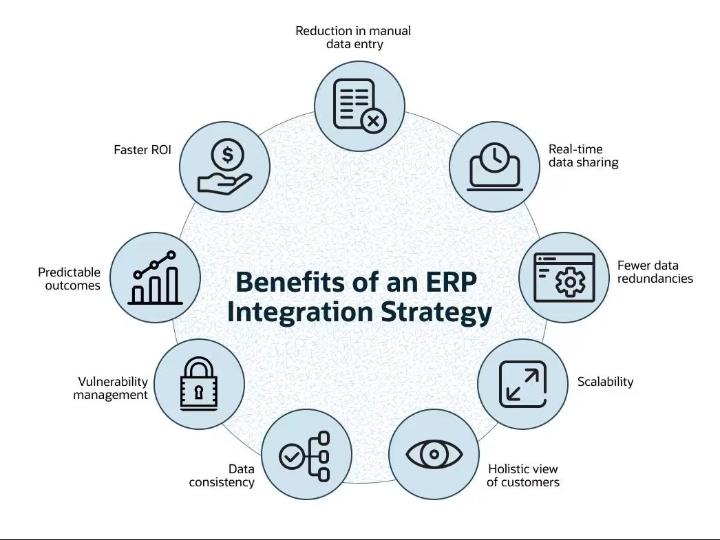
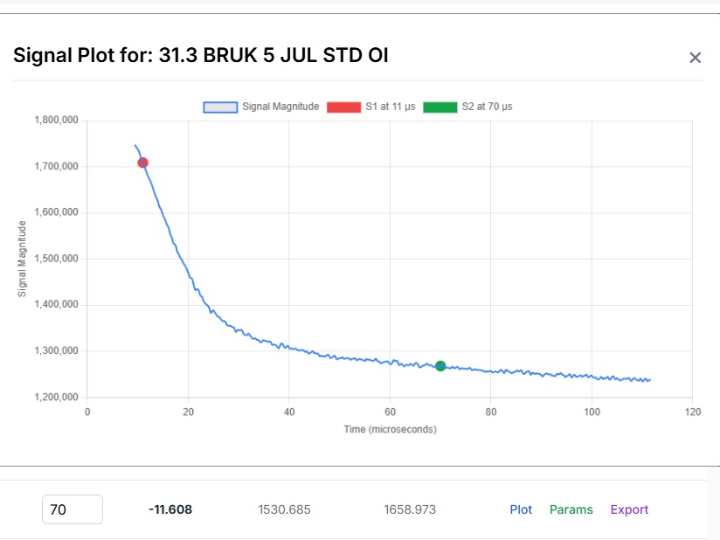
Non-Generative AI and Quality by Design: The Digital Pillar of Pharmaceutical Product Quality in the
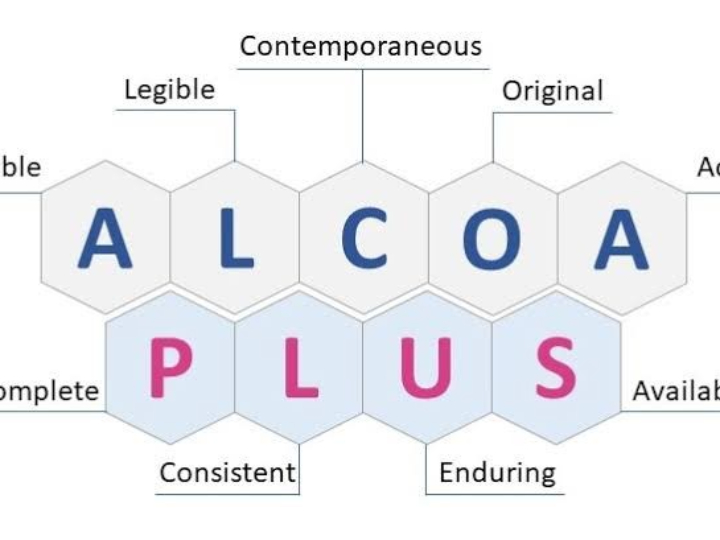
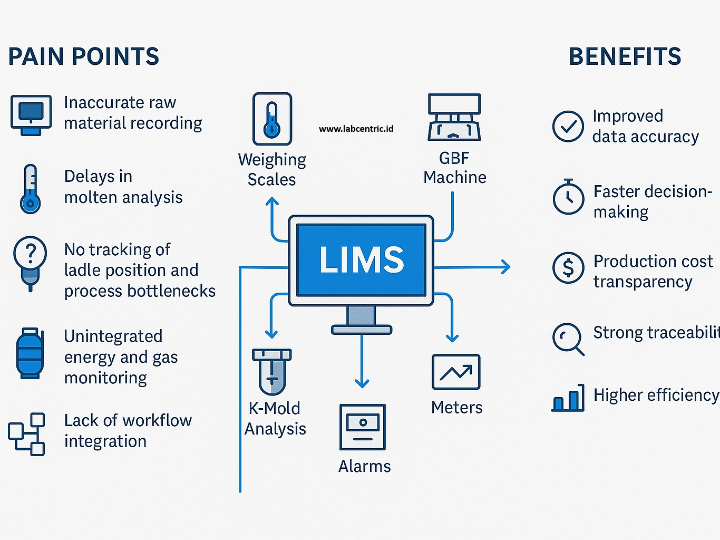
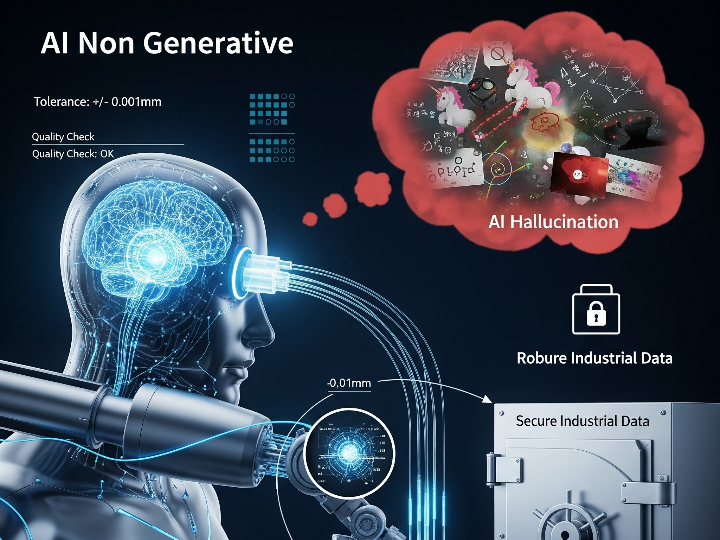
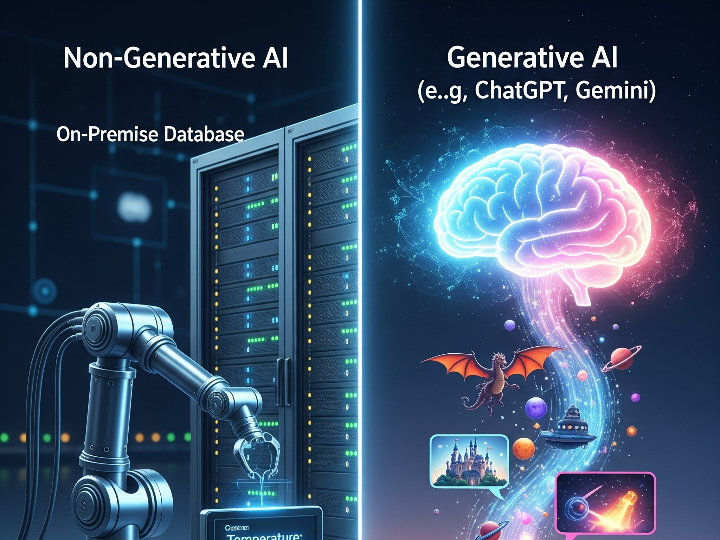
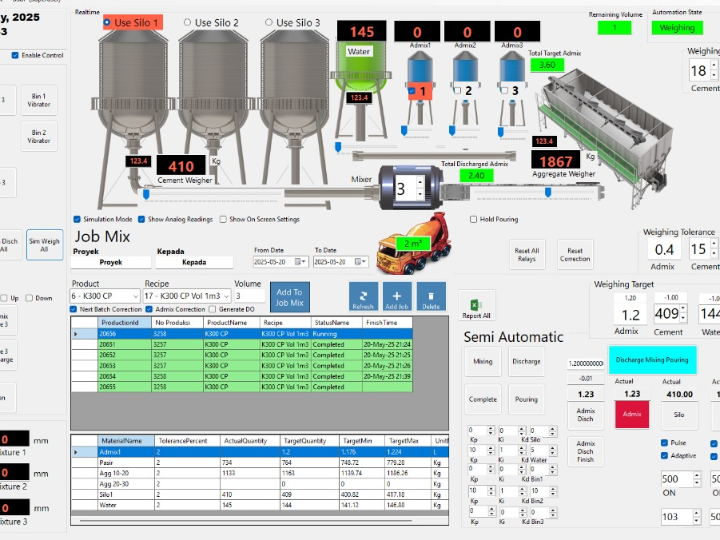
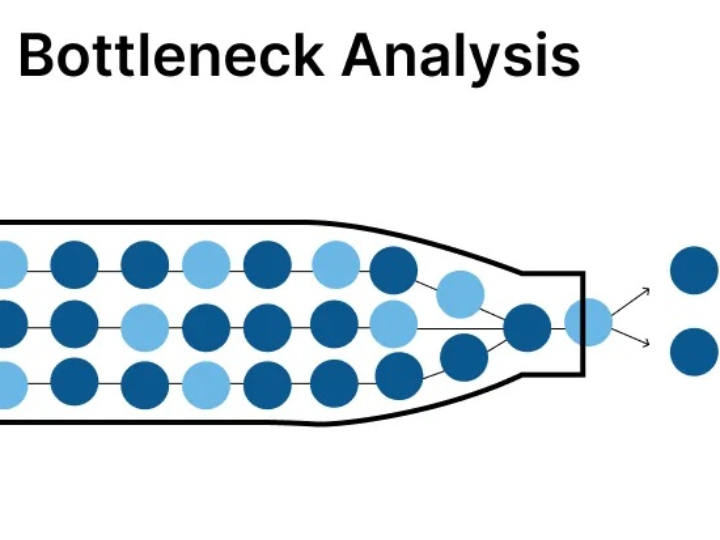
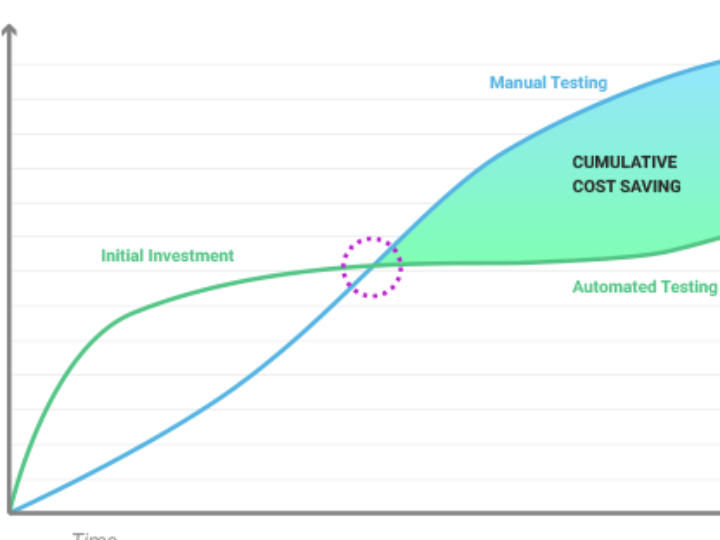
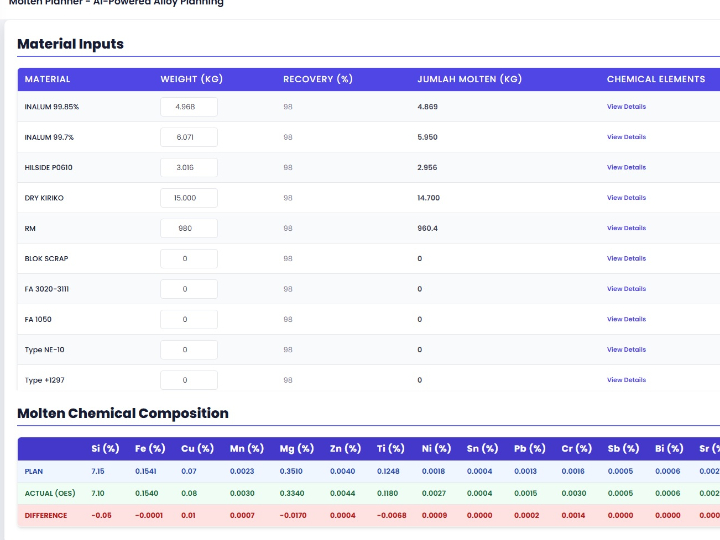
The pharmaceutical industry is a heavily regulated sector, where every product batch must guarantee consistent safety and efficacy. The Current Good Manufacturing Practice (cGMP) standard, or in Indonesia known as Cara Pembuatan Obat yang Baik (CPOB), is the operational foundation. However, in the Industry 4.0 era, demands have shifted from merely following procedures to ensuring quality through a deep understanding of the processes. This is the core of Quality by Design (QbD).