I. Introduction: Quality that is Designed, Not Just Tested
The pharmaceutical industry is a heavily regulated sector, where every product batch must guarantee consistent safety and efficacy. The Current Good Manufacturing Practice (cGMP) standard, or in Indonesia known as Cara Pembuatan Obat yang Baik (CPOB), is the operational foundation. However, in the Industry 4.0 era, demands have shifted from merely following procedures to ensuring quality through a deep understanding of the processes. This is the core of Quality by Design (QbD).
QbD is a systematic approach to development that begins with predefined objectives and emphasizes product and process understanding, and process control based on sound science and quality risk management. To effectively implement QbD, pharmaceutical manufacturers must be able to manage, analyze, and extract insights from a very large and complex volume of data.
This is where Artificial Intelligence (AI) emerges as a transformative solution. However, in a pharmaceutical environment that demands absolute data auditability and accountability, broad and unpredictable Generative AI is not sufficient. What is needed is Non-Generative AI: AI that is focused, predictive, and tightly bound to the source data.
This article will thoroughly examine why Non-Generative AI is becoming a main pillar in supporting every element of QbD, and how its implementation through an integrated Laboratory Information Management System (LIMS) can revolutionize the quality of your pharmaceutical products.
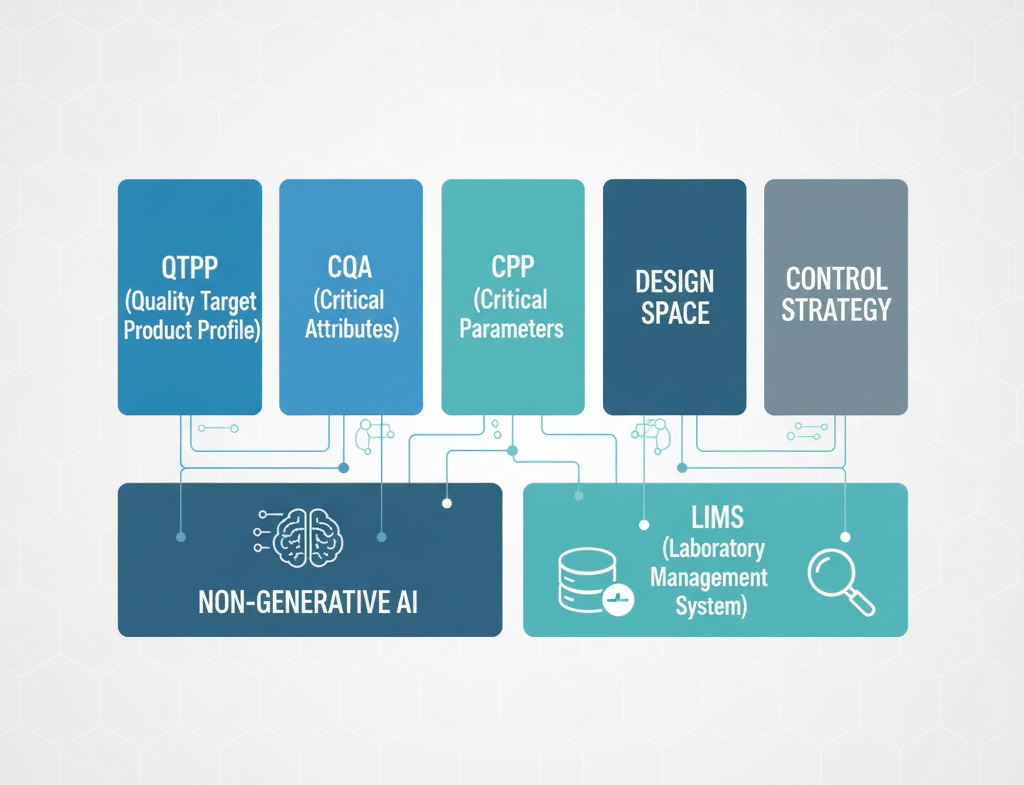
II. Understanding the Basic Pillars of Quality by Design (QbD)
QbD is not just a term; it is a framework composed of several key elements. Non-Generative AI plays a central role in optimizing each of these elements:
1. Quality Target Product Profile (QTPP)
This is a prospective summary of the ideal quality characteristics that a drug product should achieve. AI assists in reviewing clinical and market data to validate and prioritize which QTPP characteristics are most essential.
2. Critical Quality Attributes (CQA)
CQAs are physical, chemical, biological, or microbiological properties that must be within defined limits to ensure product quality. Non-Generative AI can analyze historical data to identify which CQAs are the most volatile and most affected by process variables.
3. Critical Process Parameters (CPP)
CPPs are process variables whose variation impacts the CQA. Non-Generative AI functions as an intelligent correlation engine, linking subtle changes in CPPs (such as mixing temperature, flow rate) with fluctuations in critical CQAs.
4. Design Space
This is the multidimensional combination and interaction of tested CPPs that provides assurance of product quality. Defining the optimal Design Space is a highly data-intensive task—a task perfected by the analytical capabilities of AI.
5. Control Strategy
The Control Strategy is a planned set of controls, derived from product and process understanding, to ensure product quality. AI provides the predictive foundation for this Control Strategy.
III. Fundamental Advantages of Non-Generative AI in the Pharmaceutical Environment
Why must the pharmaceutical industry choose Non-Generative AI and move away from the unpredictability of Generative AI? The answer lies in regulation and the demands for data integrity.
1. Auditability and Process Validation
In pharmaceuticals, every decision affecting product quality must have a clear audit trail that can be traced back to validated source data.
-
Non-Generative AI (Predictive): These AI models (such as multivariate regression, classification, or clustering models) produce deterministic output. This means if you input the same data, you will get the same output, which is an absolute prerequisite for Computer System Validation (CSV). You can trace why the AI made a specific prediction (e.g., through feature importance analysis).
-
Generative AI: Its output is stochastic (probabilistic) and often unexplainable (black-box), making it nearly impossible to audit or validate according to FDA 21 CFR Part 11 or CPOB/BPOM standards.
2. Data Security and Integrity (ALCOA+)
The ALCOA+ principle (Attributable, Legible, Contemporaneous, Original, Accurate, plus Complete, Consistent, Enduring, Available) is the golden standard for pharmaceutical data integrity.
Non-Generative AI integrated with LIMS ensures that the data used for model training and predictive analysis is:
-
Attributable: Data directly from the instrument (via integration) or from LIMS, recording who did what.
-
Contemporaneous & Accurate: Data is acquired and analyzed real-time, eliminating the risk of human error from manual entry.
Because Non-Generative AI only works with the data presented, it does not risk "fabricating" data or facts (hallucination), a problem often encountered with Generative AI.
IV. The Role of Non-Generative AI in Optimizing QbD Pillars (In-Depth Analysis)
1. Refining Design Space Through Multivariate Analysis
Finding the optimal limits of the Design Space requires sophisticated statistical capabilities that surpass human operator capacity.
-
Techniques Used: Non-Generative AI utilizes techniques such as Partial Least Squares (PLS), Principal Component Analysis (PCA), and other Multivariate Regression Models.
-
How It Works: LIMS consolidates all data: raw material data, process data (CPP), and final quality data (CQA). The AI then processes this data to:
-
Outlier Identification: Find batches that failed or almost failed, then analyze the unique CPP combination in those batches.
-
Correlation Modeling: Create a mathematical map that shows how simultaneous changes in two or more CPPs (e.g., increasing temperature while reducing mixing time) will affect critical CQAs (e.g., content uniformity).
-
-
Concrete Benefits: With AI, the R&D team can simulate thousands of CPP scenarios virtually in seconds, drastically reducing the time and cost of physical experimentation, and establishing the most robust and lean Design Space.
2. Predicting CQA in Real-Time and Process Analytical Technology (PAT)
One of the biggest breakthroughs of QbD is the shift from Quality by Testing (testing after the product is made) to Quality by Monitoring (testing and verifying as the product is made). This is realized through Process Analytical Technology (PAT).
-
AI and PAT Integration: PAT instruments (such as NIR, Raman Spectroscopy) generate highly complex spectral data in real-time processes. Non-Generative AI is the only tool capable of:
-
Acquiring this spectral data through LIMS/integration.
-
Processing it using chemometrics models.
-
Predicting CQAs (e.g., moisture content, tablet hardness, active ingredient concentration) in-line (on the production line).
-
-
Control Strategy Implications: This real-time prediction enables Feedback Control. If the AI predicts a CQA will drift out of tolerance limits in the next 30 minutes, the control system (via SCADA or DCS) can automatically adjust the relevant CPP (such as granulator speed) to bring the CQA back to target, before the batch is compromised.
3. Automating Decision Making for Non-Conformance Response
Non-Generative AI can go beyond data analysis and enter the realm of automatic decision-making in the context of Non-Conformance or NG (Not Good) products.
-
CBR System (Case-Based Reasoning): This feature can be implemented in AI-supported LIMS. Every time a batch experiences non-conformance, the AI records all process data, lab results, and the corrective action taken.
-
Automated Response: When a new batch shows a CPP/CQA pattern similar to a historical non-conformance case, the AI can automatically recommend the most successful Digital SOP (Standard Operating Procedure) based on previous cases.
-
Benefits: Ensures consistent, fast, and evidence-based response to quality issues across the entire plant, meeting the CPOB demands for operational consistency.
V. LIMS and AI Integration: The Bridge to Quality
The advantages of Non-Generative AI cannot be achieved without a reliable and structured data collection infrastructure. This is the primary role of LIMS (Laboratory Information Management System) and instrument integration.
1. LIMS as the Single Source of Truth
LIMS is the central hub that collects, manages, and standardizes all lab data. This ensures that:
-
Clean and Structured Data: AI requires clean data. LIMS ensures data is organized in a uniform format, ready for processing by the AI model.
-
Full Integration: A modern LIMS must be integrated not only with advanced instruments via network connections but also with older or offline instruments (such as through Labcentric's LIMS Stick solution). The LIMS Stick functions as a data security bridge, ensuring that older instrument data can be acquired automatically and securely, allowing this historical data to be included in the AI model.
2. The Need for Automated Data Preparation
Data from laboratories and production is often raw, noisy, or incomplete. The largest part of AI implementation time is Data Preparation. LIMS, especially those with integrated AI modules (such as Clara), can automate:
-
Handling of missing values.
-
Data normalization.
-
Integration of data from various sources (LIMS, ERP, SCADA) into a single dataset ready for AI analysis.
VI. Implementation Challenges and Cultural Preparation
Despite its major benefits, the application of Non-Generative AI in the context of QbD has challenges that must be overcome, particularly in Indonesia:
1. AI Model Validation
Pharmaceutical regulations demand that AI predictive models must also be strictly validated and documented, similar to the validation of the LIMS software itself. This requires specific statistical and regulatory expertise.
2. Cultural Transformation
The transition from operators who rely on intuition or manual procedures to operators who trust predictive recommendations from AI requires intensive training and a shift in the culture of quality risk management.
3. Initial Data Quality
The old saying, "Garbage In, Garbage Out" (GIGO), is highly applicable. If the historical data fed into the AI is inaccurate (due to manual recording, input errors, or incorrect time stamps), the AI model will generate invalid predictions. This is why investment in robust instrument integration (like that offered by Labcentric) must be made upfront.
VII. Conclusion: The Future of Pharmaceutical Quality
Quality by Design (QbD) is the gold standard for ensuring the quality, safety, and efficacy of pharmaceutical products. To truly master QbD, pharmaceutical manufacturers can no longer rely on spreadsheets or limited data analysis.
Non-Generative AI offers the predictive analytical power and control absolutely necessary to:
-
Establish a strong and cost-effective Design Space.
-
Implement a Control Strategy through real-time CQA prediction (PAT).
-
Meet ALCOA+ and CPOB compliance standards.
By choosing an AI solution specifically designed for a regulated industrial environment, and supported by a robust data integration system (LIMS), pharmaceutical companies can move from merely complying with rules to defining new quality standards.
Want to Master QbD and Integrate AI with Full Compliance?
We at Labcentric understand the strict demands of the Indonesian pharmaceutical industry and CPOB standards. Our solutions, powered by the non-generative Clara AI, are seamlessly integrated with your LIMS, complemented by unique hardware solutions like the LIMS Stick to guarantee data integrity from all instruments, old and new.
Contact us today for an in-depth consultation on your pharmaceutical laboratory digitalization strategy and see how AI can become your best Quality Engineer.