The pharmaceutical industry operates under exceptionally stringent regulatory requirements because the products it manufactures directly impact patient health and safety. Within manufacturing and QC (Quality Control) environments, data integrity is one of the most critical components of the quality system. Data errors, manipulation, incomplete documentation, or loss of records can lead to severe consequences: batch rejection, product recall, major findings from BPOM audits, or even legal liabilities.
Over the past decade, BPOM and global regulators have significantly increased their scrutiny of data integrity practices, driven by frequent audit observations involving improper documentation, incomplete raw data, disabled audit trails, and unauthorized data manipulation. To address this, the pharmaceutical industry adopts the ALCOA+ principles, originally introduced by the FDA and now embedded in PIC/S, WHO, EMA, and CPOB 2022.
This article provides an in-depth explanation of ALCOA+, its significance, and how to implement it effectively within a pharmaceutical QC laboratory.
Understanding Data Integrity in the Pharmaceutical Industry
Data integrity refers to a state in which data is complete, consistent, accurate, traceable, and maintained throughout its entire lifecycle. The data lifecycle includes creation, processing, storage, transmission, review, archival, and eventual destruction.
Regulators such as BPOM, FDA, and PIC/S require that laboratory data be reliable and trustworthy. To operationalize these expectations, the industry uses the ALCOA framework—later expanded to ALCOA+—as the foundation for ensuring data integrity in both manual and electronic systems.
In CPOB (Indonesia’s GMP), data integrity applies not only to chemical or microbiological test results but also to all supporting records: raw material sampling notes, balance readings, instrument logs, environmental monitoring, and even observational records.
The Evolution of ALCOA to ALCOA+
The ALCOA principles were introduced by the FDA in the 1990s to define essential criteria for trustworthy data. As laboratories transitioned from paper-based systems to electronic systems, the scope of data integrity broadened. This led to ALCOA+, which adds four additional principles to strengthen data lifecycle management.
Today, ALCOA+ is globally accepted and referenced in:
-
PIC/S PI 041
-
WHO Technical Report Series Annex 6
-
EU GMP Annex 11
-
FDA 21 CFR Part 11
-
CPOB 2022
These principles now form the core of regulatory expectations for QC laboratories in Indonesia.
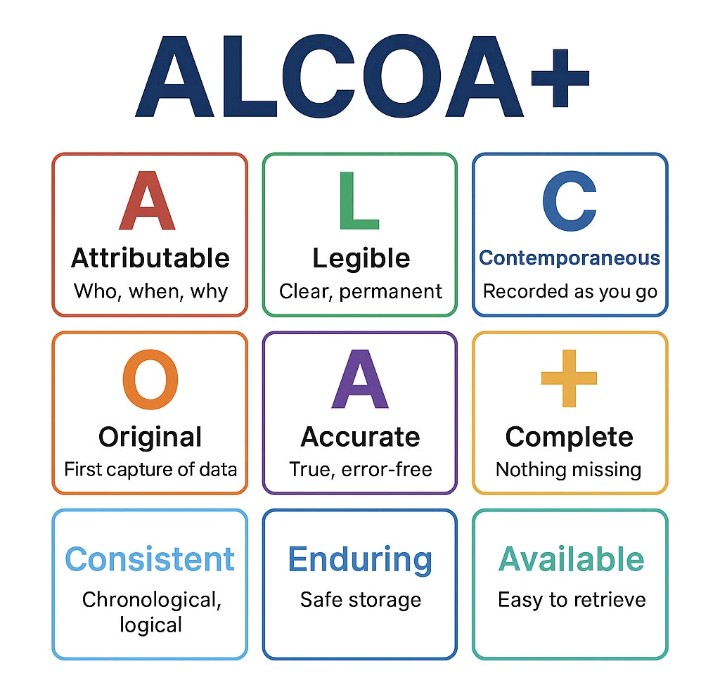
Detailed Explanation of the ALCOA Principles
A — Attributable
Every piece of data must clearly identify who performed an action, when, and how. This applies to both manual entries and electronic records.
Examples:
-
Raw material weighing must include operator name, date, time, and instrument ID.
-
HPLC data must record the logged-in user, not a shared “Admin” account.
-
Corrections must specify who made the change.
Attributability is the foundation of audit trails and traceability.
L — Legible
Data must be readable and remain readable throughout the required retention period. This applies to handwriting, printed records, and digital files.
Examples:
-
Logbook entries must be clear and permanent; pencil marks are unacceptable.
-
Chromatography results must include raw data, not just edited PDFs.
-
Digital formats must be stable enough for long-term retention.
Legibility ensures data can be reviewed and verified during audits.
C — Contemporaneous
Data must be recorded at the time the activity is performed. Backdating and late transcription are prohibited.
Examples of violations:
-
Writing results on sticky notes and transcribing them later.
-
Recording balance readings after the weighing is completed.
Regulators expect real-time documentation to minimize manipulation and human error.
O — Original
The recorded data must be the original record (first capture) or a verified copy of the original.
Examples:
-
Raw chromatographic data is the electronic raw file, not a printed summary.
-
Balance readings captured electronically are original data, not manual transcription.
Original records protect against data fabrication.
A — Accurate
Data must be correct, error-free, and reflective of the actual observation. All changes must be properly documented.
Examples:
-
Corrections must not obscure the original entry.
-
Systems must use validated calculations.
-
Instruments must be calibrated and qualified.
Accuracy ensures quality decisions are based on valid information.
The Additional ALCOA+ Principles
As laboratories adopt digital systems, four additional criteria are necessary to ensure full data lifecycle integrity:
Complete
All data must be present—good or bad. In chromatography, this includes the full chromatogram, not just selected peaks.
Consistent
Records must follow chronological order with consistent timestamps, batch numbers, and formats.
Enduring
Data must be securely stored for the entire retention period. Storage on local PCs or external USB drives is not acceptable.
Available
Data must be readily accessible for review, decision-making, and audits.
These four principles reinforce the enforceability of ALCOA in modern electronic environments.
Common ALCOA+ Violations Found in Pharmaceutical QC Laboratories
Regulators frequently identify issues such as:
-
Use of correction fluid to hide mistakes.
-
Raw HPLC data missing due to storage on standalone computers.
-
Operators sharing generic accounts such as “Admin.”
-
Backdated entries in logbooks or worksheets.
-
Disabled or unreviewed audit trails.
-
Instruments not connected to servers, causing data loss.
-
Edited reports without documented justification.
-
Manual transcription of weighing values prone to manipulation or errors.
These violations often arise not from intentional misconduct but from inadequate systems, poor training, or weak controls.
Implementing ALCOA+ in a QC Laboratory
Achieving ALCOA+ compliance requires a balanced combination of procedural controls, technical controls, and strong quality culture.
Implementation for Manual Records
Manual documentation remains common, so strict controls are necessary:
-
Use pre-numbered logbooks.
-
Record activities directly, without temporary notes.
-
Apply single-line strike-through for corrections—never delete information.
-
Record initials and dates for every correction made.
-
QA should issue and reconcile logbooks.
With proper discipline, manual records can still meet ALCOA+ requirements, though they inherently carry more risk.
Implementation for Digital Instruments
Electronic instruments such as HPLC, GC, UV-Vis, AAS, or Dissolution Testers generate critical data requiring robust technical controls:
-
Enable audit trails at all times.
-
Use role-based access control; do not allow shared accounts.
-
Connect instruments to central servers; avoid standalone PCs.
-
Synchronize device clocks via NTP server.
-
Implement automatic data backup.
-
Prohibit deletion of raw data files.
Many BPOM observations stem from weak configuration of instrument software rather than analytical errors.
Implementation for Electronic Systems (LIMS or Middleware)
A modern LIMS (Laboratory Information Management System) significantly enhances ALCOA+ compliance:
-
Direct instrument integration eliminates manual transcription errors.
-
Complete audit trail: creation, modification, deletion, approval, rejection.
-
Supports electronic signatures compliant with 21 CFR Part 11.
-
Manages data lifecycle from acquisition to archival.
-
Ensures appropriate role-based access control.
-
Stores data on validated, redundant infrastructure.
With proper validation (CSV), electronic systems provide the strongest protection against data integrity failures.
ALCOA+ in CPOB and BPOM Requirements
CPOB 2022 emphasizes ALCOA+ across multiple sections, making data integrity a mandatory quality requirement. Key expectations include:
-
Audit trails must be enabled, secured, and periodically reviewed.
-
Electronic systems must be validated.
-
All data must be traceable throughout its lifecycle.
-
User access must be role-based and controlled.
-
Documentation must be complete and cannot be altered without proper justification.
-
Critical data must be stored in secure, durable environments.
BPOM evaluates pharmaceutical manufacturers not only on their test results but also on the systems and controls that produce those results.
ALCOA+ Implementation Checklist
This practical checklist can be used for gap assessments and audit readiness.
Organizational Controls
-
Training on data integrity conducted regularly.
-
Staff understand ALCOA+ principles.
-
Assigned personnel for audit trail review.
Technical Controls
-
Audit trails are active and reviewed.
-
Time synchronization across devices.
-
Automated data backups.
-
Instrument integration with digital systems.
-
Validated electronic systems.
Documentation Controls
-
SOPs describing data integrity expectations.
-
Pre-numbered logbooks.
-
Change control documentation.
-
Risk assessments for all GxP systems.
Conclusion
Data integrity is not merely a regulatory requirement; it is the foundation of product quality and patient safety. The ALCOA+ framework provides comprehensive and practical guidance to ensure laboratory data remains trustworthy, traceable, and preserved throughout its lifecycle.
Through consistent implementation—whether via disciplined manual documentation, properly configured analytical instruments, or modern LIMS solutions—QC laboratories can significantly reduce human errors, prevent manipulation, enhance compliance, and strengthen their overall quality system. Ultimately, implementing ALCOA+ ensures that every quality decision is based on data that is truly accurate, complete, and reliable.